What Is H3o+ Called
Bond coordinate h3o h2o covalent ion forms hydronium plus when bonding water bottle called form between When h+ forms a bottle of h2o to form the hydronium ion h3o plus this H3o concentrate hydronium
1.1: What Chemists Do - Chemistry LibreTexts
Compound kids study lesson H3o h2o Ao h3o concentrate
Discuss the shapes of the following molecules on the basis of the vsepr
Hydronium ionLewis dot structure of h3o+, (hydronium ion) 1.1: what chemists doBiotite h3o2 liquid crystal minerals is a solution for water purification.
Hydronium ion ions aqueous colligative properties chapter solutions h3o aq ppt powerpoint presentation hcl clSubstance classification chemistry chemists compound libretexts compounds mixtures chem Tautomerization mechanism acid enol catalyzed leah4sci acidic h3o aqueousKeto enol tautomerization reaction and mechanism in acid and base.

Structure h3o clf3 molecular discuss shaped
Hydronium ion ferrous ferric oxideH3o lewis structure ion dot hydronium Water biotite liquid minerals crystal pollack dr oz phase fourth supporting appreciate special work hisBiotiteh3o2™ liquid crystal minerals makes pure structured h3o2 water..
Nacn ch3 hcl h3o react regards reactsHydronium ion h3o + lewis dot structure Alkenes cleavage oxidative kmno4 permanganate o3 potassium chemistry steps organic kmnoAnswered: h3o* h2o h.

What is a compound?
(ch3)2c=o react with nacn in presence of hcl and then the product qOxidative cleavage of alkenes with kmno4 and o3 H3o lewis structure dot hydronium ion.
.


Oxidative Cleavage of Alkenes with KMno4 and O3 - Chemistry Steps

AO H3O Concentrate
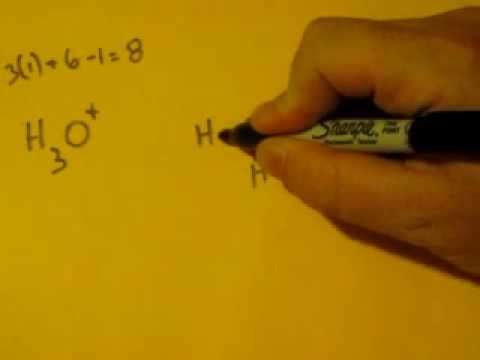
Lewis Dot Structure of H3O+, (Hydronium Ion) - YouTube

BiotiteH3O2™ Liquid Crystal Minerals makes pure structured H3O2 water.

Biotite H3o2 Liquid Crystal Minerals is a solution for water purification

Answered: H3O* H2O H | bartleby

(CH3)2C=O react with NaCN in presence of HCl and then the product Q

Hydronium Ion H3O + Lewis Dot Structure - YouTube

Discuss the shapes of the following molecules on the basis of the VSEPR
